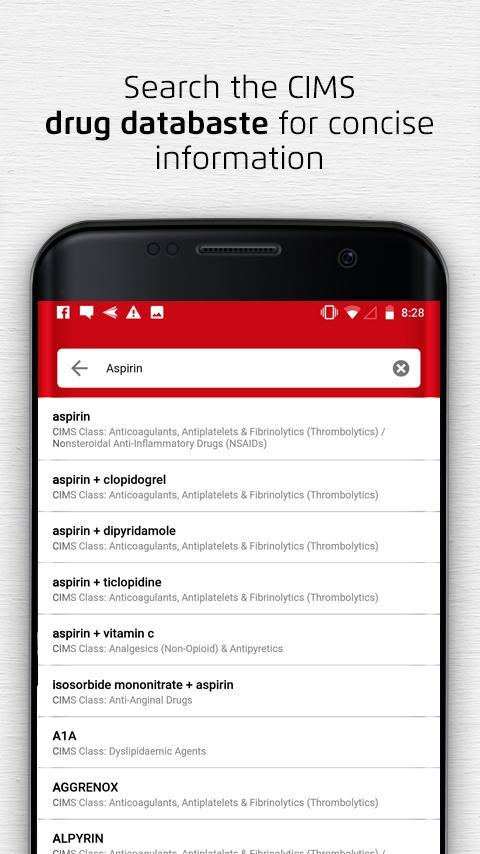
- Search drug information, interaction, images & medical diagnosis. The most comprehensive database of medicines available in China, Hong Kong, Taiwan, Malaysia, Singapore, Philippines, Vietnam, Thailand, Indonesia and India.
- Search Drug Information, Images & Medical News CIMS India. Advanced Search. Top medical news. Blue light-blocking glasses may be a gateway to better sleep 05 Dec 2020 Wearing blue light-filtering glasses for at least 2 hours before bedtime may be the solution to a better night's sleep and also a better performance the following.
- Www.kvetinyuelisky Mims Drug Handbook 2015 - web-server-04.peakadx.com 2009 Nurses Drug Handbook PDF, EPUB EBOOK Mims Pathogenesis Of Infectious Disease Fifth Edition PDF Cims drug book 2012 free download pdf Geriatric Dosage Handbook PDF, EPUB EBOOK Critical Care Assessment Handbook 1e PDF 2010 Nurses.
- For over 50 years, MIMS has provided trusted and relevant clinical information for over two million healthcare professionals in Asia. Designed for busy individuals on the go, the MIMS app is a convenient one-stop clinical reference that provides healthcare professionals with the clinical decision support they need at the point of care. The MIMS Mobile app for Android™/ IOS™ is available.
- Cims Drug Book 2019 Pdf Free Download
- Cims Drug Book 2020 Pdf Download
- Mims Drug Book Pdf
- Cims Drug Book 2018 Pdf Download
- Cims Drug Book 2018 Pdf Free Download
- Cims Drug Book 2017 Pdf Download
with world-class innovation
We deliver best outcomes through a multidisciplinary and integrated team approach. Our expertise draws on the skills of our entire team across Asia Pacific – exceptional scientific content creation with strategic marketing insights, impactful creatives, seamless project management, rigorous editorial standards and strict regulatory compliance.
Entire MIMS Manual (pdf) manualv1-22.pdf: 4.46 MB: Printer-friendly version.
- Multi-country drug information databases (13 countries in Asia) Powered with CIMS drug information (Drug Information, Drugs A to Z, Companies A to Z, Therapeutic Class, Calculators)
- Enhanced with Search Media (Medical search engine) and Google (World Wide Web search) .
- Innovative marketing channel .
- Cost-effective internet strategy .
- Prime Sight
- Home Page Banner Advt
- Poll ( Feedback )
- Direct Emailer Service
- Newsletter .
Visit mims.com- The Most Powerful Drug Search Engine
Most advanced Drug Search Engine for (iOS & Android, Tablet).
CIMS Mobile App offers drug search, Clinical news, General news, special reports, multimedia, calculators, medicalevents, QR scanner, bookmarks and also information on Disease’s signs and symptoms, diagnosis, treatment, management, News & CME, references.
iOSAPP store
cims India – Drug Information, Disease, News .
AndroidGoogle play
cims India – Drug Information, Disease, News .
CIMS INTEGRATED provides a Clinical Decision Support Knowledge bases to help healthcare professionals in electronic prescribing and dispensing environment make better informed prescribing & therapeutic decisions
CIMS INTEGRATED provides a comprehensive knowledge base of locally approved drug information powered with internationally referenced clinical decision support tools. It`s embedded in the clinical application system, supplying up to date drug information and interacting intelligently with the patient profile to maximize healthcare professionalism time.
CIMS INTEGRATED enables healthcare professionals to improve the quality of patient care in the area of medication management. This solution is a fully customizable drug database that can be mapped to any hospital formulary and integrated into a prescribing system to double up as the backbone of the inventory management process.
CIMS* has been the hallmark of drug information since 1978 in India. An advanced Drug Information System, CIMS continues to evolve to meet the emerging requirements and needs of healthcare professionals.
CIMS has developed tools and knowledge base for integration into clinical applications for decision support, helping healthcare professionals make informed decisions at the point of care. Introducing most widely used Product “CIMS Integrated” since it is currently being used by 11,000 + customers across Asia Pacific regions.
CIMS INTEGRATED provides a Clinical Decision Support Knowledge bases to help healthcare professionals in electronic prescribing and dispensing environment make better informed prescribing & therapeutic decisions.
CIMS INTEGRATED provides a comprehensive knowledge base of locally approved drug information powered with internationally referenced clinical decision support tools. Its embedded in the clinical application system, supplying up to date drug information and interacting intelligently with the patient’s profile to maximize healthcare professionals time.
CIMS INTEGRATED enables healthcare professionals to improve the quality of patient care in the area of medication management. This solution is a fully customizable drug database that can be mapped to any hospital formulary and integrated into a prescribing system to double up as the backbone of the inventory management process.
Drug Information:
*The Drug-Info module delivers timely regularly updated prescribing information on pharmaceutical products. This module comprises of independently compiled generic monographs and list of global approved generic master
*CIMS Database enables quick retrieval of independently compiled CIMS essential monographs
*Independently compiled molecule monographs

*Available for over 1,800 molecules
*Each Drug Monograph features key prescribing information for more informed decision making during prescription.
a) Overview –
CIMS Integrated product helps & assist clinicians in making better & more informed choices & decision., CIMS has a range of evidence-based Clinical Decision Support modules developed by our expert team of pharmacists. Independent of the manufacturer’s product information, these modules are developed using international primary and tertiary references across the Globe.
These Modules include the ability to check for drug interactions between two or more drugs, against a patient’s health condition/s or allergies, or checking if two or more drugs contain the same active ingredient/s.
b) Feature: Severity level of interaction
Level of documentation to support the interaction
Description of probable mechanism of the interaction
Pharmacological action of each interacting drug
Recommended action
International references
Clinical information – relevant co-morbidity risk factors, explanation of appropriate management; Case discussions – where important for urgent clinical understanding
Precautions, indicating the most appropriate and relevant courses of action to be pursued concerning the interaction References, indicating the relevant primary literature for the interaction
FormatAvailable in the API formats for integration into clinical software applications.
Different Clinical Decision Support Module:
The Drug Alert module processes drug-drug interaction checks. An interaction warning displays essential information after checking for an interaction between two drugs The Drug Interactions module warns healthcare providers if drugs being used by a patient or prescribed to a patient may interact with each other. An interaction record in the Drug Interactions module contains a description of the interaction, what could be expected if there is an interaction, severity of the interaction, strength of the supporting evidence and precautions needed. This helps the healthcare professional and, eventually, the patient to avoid treatment failures, adverse effects and toxicities that could be life threatening.
Examples:
- itraconazole + phenytoin
- warfarin + fenofibrate
- phenytoin + carbamazepine
- carbamazepine + chlorpromazine
- phenytoin + diazoxide
The Drug Allergy Alert module enables the healthcare professional to process drug allergy checks at the point of care by comparing a patient’s drug allergy profile and the current medication regime, against the active ingredients in the medications about to be ordered.
Patients can have allergic reactions to molecules of varying degrees of severity, ranging from a rash to anaphylactic shock. This important Clinical Decision Support tool enables a practitioner to be automatically alerted when a prescribed medication contains active ingredients that are known to cause an allergy in their patient.
Examples:
- penicillins + cephalosporins
- heparinoids + heparins
- salicylates + tartrazines
The Drug Health Alert module is used in conjunction with the patient’s profile for stored medical conditions and subsequently for potential contraindications with the prescribed medication. The database currently supports WHO ICD10 and can be cross-referenced to several standard disease classification systems worldwide. The alert returns information on the severity, the level of documentation and a description of the contraindication. All information is internationally referenced.
The Drug Disease Contraindications Clinical Decision Support Module (CDSM) is a tool that alerts the healthcare provider if prescribed or administered drug(s) interacts with the patient’s illness. An interaction record in this CDSM contains a description of the interaction, what could be expected if there is an interaction, severity of the interaction, strength of the supporting evidence and precautions needed. This helps the healthcare professional and, eventually, the patient to avoid adverse events and toxicities that could be life threatening.
Examples:
- digoxin + sick sinus syndrome
- ciclosporin + melanoma
- ciclosporin + renal impairment
Duplicate Alert Module provides an alert if the medicine to be prescribed, dispensed or administered has an identical therapeutic function to another medicine already prescribed to the patient.
The alert algorithm is based on the ATC (Anatomical Therapeutic Chemical) classification system for the drugs from the World Health Organization. For example, if prescribing a medication that contains codeine this module will check existing prescriptions for that patient and warn the prescriber if one of these contains codeine as well.
Examples:
- omeprazole + ranitidine
- omeprazole (proton pump inhibitors group) and ranitidine (H2 antagonists group) are both indicated for GI ulceration.
- OMEZ + LANZOL
- OMEZ contains omeprazole and LANZOL contains lansoprazole. Both are proton pump inhibitors.
CIMS provides a range of products – to a wide cross section of the healthcare market. From hospitals to aged care, medical practitioners, Clinics to pharmacists to dentists, health services, Software developer to border protection authorities – we cover it all!
1. Hospitals:
There are millions of General Practice surgeries in India that are made up of corporate, small group and private practices. Working within these are a mix of General Practitioners and Specialists, Practice Nurses, Nurse Practitioners and other healthcare professionals such as Pharmacists and Podiatrists.
CIMS understands the diverse needs of those who work in this environment and this understanding ensures we provide India ’s most complete medicines reference together with patient counselling tools and evidence based drug interactions in the most accessible manner.
2. Clinics:
There are many General Practices that are made up of corporate, small group and private practices. Working within these are a mix of General Practitioners and Specialists, CIMS understands the diverse needs of those who work in this environment and this understanding ensures we provide most complete medicines reference and evidence based drug interactions in the most accessible manner. Ensuring you can access your CIMS within your prescribing software which is being used in the Clinics.
3. Pharmacies:
With the introduction of ever more complex drug therapies and the drive to reduce preventable errors, rapid access when needed to relevant information and trusted medicines information is crucial. Using CIMS Integrated, pharmacists can be confident that they have the most current medication resources together with patient and consumer information. CIMS Integrated provides;
A source of current Drug Information.
A Drug Interactions reference and An evidence-based reference work on complementary and alternate medicines
When you’re dispensing at a rate of knots you need to know the information you want is where you need it, when you need it, that’s why we offer options as to how you can access your CIMS. CIMS Integrated can be integrated into your dispensing software so you can be confident on the drug interactions and the most updated drug information.
4. Software Developers:
Our partners understand that the CIMS data they integrate comes from a source their users trust and adds value to the work they are doing with common end customers like hospitals, clinics or retail pharmacy stores. CIMS Integrated reference data and our clinical decision support solutions are incorporated within many of World’s leading clinical software solutions like Hospital Information Systems (HIS), Electronic Medical Record (EMR), …etc. CIMS works with our partners before, during and after the integration of our data base and decision support modules into their software.
Our team of experienced clinical editors, technical staff and business development managers will work closely with you from the outset so we can fully understand your needs and recommend the most suitable integration option. We work with your development team to integrate the products and provide you with editorial support to ensure your integration is able to reduce risk. Being a CIMS partner really is just that and we‘ll be with you every step of the way before during and after you launch to support your growth and end user experience.
To apply to become a CIMS Partner, contact our Business Development Manager who works in and understands your market.
Support:
We take support seriously and strive to ensure that we can give you the answers to your questions quickly and respectfully.
CIMS provides technical and clinical support for CIMS Integrated products Monday to Friday, 9am to 5pm IST except for India public holidays.
Request for Demo -
Kindly contact @ 022-66122600, or email directly at support@cims.co.in
Advertising banners , prime sights, prime link, pop-up
Monthly enewsletter – customized emailers to specialized healthcare audience
eNewLetters
Preferred poll survey on cimsasia website – conducting survey by sending eflerys to specific audience
“CIMS” is India's leading provider of Multi-Channel Solutions for Healthcare Information Needs.
- CIMS is the most up to date and comprehensive source of locally approved drug information, and internationally referenced and clinically reviewed
- “CIMS” is the flagship product in the field of Drug Information System, which is successfully serving the medical professionals and with utmost dedication has entered its 39th Year of Leadership. For the past almost 4 decades CIMS has been the undisputed leader as the “Updated Prescriber's Hand-Book' of choice.
- CIMS provides essential clinical decision support to help optimise clinician’s time and improve the quality of patient care.
- CIMS provides medication management at the point of care.
- CIMS on quarterly basis provides refreshed drug information.
- “CIMS” is committed to a huge expansion and diversification as per our global corporate policy.
- CIMS has been rated by ORG-MARG in their previous survey as the leader in its class having the 'Top of Mind' recall value among doctors in India.
- Powerful tool for Product launch.
- Booklet circulated with CIMS containing Product information in form of a Monograph – Indication Focused.
- Gives Branding Options along with CIMS Endorsement.
CIMS Annual is a comprehensive drug database and it is updated Prescribers Hand-Book.
- CIMS Annual provides updated clinical information and updates from the Drug Controller General of India.
- CIMS Annual is a compendium abundant in pharmaceutical brand’s information and generics monographs.
- CIMS Annual provides essential clinical decision support to help optimise clinician’s time and improve the quality of patient care.
CIMS Annual provides :
- Medication management at the point of care.
- Information on Treatment Guidelines
- Management of disease information
- “Top of the mind” recall
IDR Triple-i, is an unique format has been conceived and created for the Point of Formulary
- Drug information at the ease of search.
- It has a triple index which makes it more users friendly.
- i-Therapeutic section index
- i-Company index
- i-Generic & Brand index .
The valuable inputs and suggestions are received from the Pharmaceutical Industry have helped the publication emerge with a platter of unmatched features.
JPOG equip readers with a wide range of practical information on woman & child care.
- Trusted for its high academic standards & editorial quality since 1974.
- Information on latest trends & development.
Super speciality journal for cardiologist, Cardiac Surgeons & Physicians
- Information on latest trends & development
- Collection of evidence and practice based flow charts
- Consolidate knowledge about disease condition
- One stop access to guidelines with detailed information on signs, symptoms, diagnoses & dosage guidelines
- More than 10 Therapeutic titles available
Example: CIMS Antimicrobial, CIMS Cardiology, CIMS Dermatology, CIMS Endocrinology, CIMS Gastroenterology, CIMS Neurology & Psychiatry, CIMS Obstetrics & Gynecology, CIMS Respiratory, CIMS Ophthalmology, CIMS Pediatrics.
CIMS is complemented by CIMS Speciality Editions. Each CIMS Speciality edition is a collection of evidence and practice based flowcharts, which consolidate knowledge about desease conditions to support effective clinical decision making.
CIMS Speciality flowcharts include signs, symptoms and diagnosis, as well as treatment options and dosing guidelines for common clinical conditions.
- Cardiology
- Cardio metabolic risk management
- Dermatology
- Endocrinology
- Gastroenterology
- Hepatology
- Infectious diseases
- Internal medicine
- Neurology
- Obstetrics & gynecology
- Oncology
- Ophthalmology
- Pediatrics
- Psychiatry
- Respiratory
- Rheumatology
- Urology
Each CIMS Specialty edition is a collection of evidence- and practice-based flowcharts, which consolidate knowledge about disease conditions to support effective clinical decision making. CIMS Specialty flowcharts include signs, symptoms and diagnosis, as well as treatment options and dosing guidelines for common clinical conditions.
Print, digital and multichannel marketing services for new and existing products, include: • Scientific and marketing content development
- Sales and marketing materials
- Product launch kits and programmes
- Detailing aids in print and electronic formats
- Newsletters and symposia highlights
Medcomm addresses up to date healthcare information needs and delivers strategic, integrated communications solutions and comprehensive reach ─ online, in print, live and via custom programs. One-stop solution for all the scientific deliverables - multi channel.
Products offered by Medcomm Division:
Medcomm helps healthcare professionals in pre-launch and launch marketing strategies, regional and local branding and product positioning strategies and in continuing medical education (CME) program planning and development . Division focuses on brand plans and solutions for the new products as well as the existing products for Doctor and Patient Education.
Point-of-care tools to assist clinicians by providing up-to-date treatment and disease management resources.
Drug listing
- Advertising opportunities
- Scientific and marketing resources
- Informative videos
- Interactive polls
Manage and organize CME/CPD accreditation through:
- Print journals
- eLearning
- Live meetings for congresses and scientific symposia
- Ad board is an advisory board comprising of 8-10 top KOLs, who are identified and recruited to form a consensus on the focused topic by building concept to bridge the gap in the treatment
- This is achieved by developing the questionnaire of relevance and then developing the draft of the outcome.
- This content of the outcome can be supported by manuscript development to publish ready for reputed journals
- It is connecting the international KOL with local doctors across regions, for Individual/group viewing by the doctors. Real time interaction with the international KOL can be done by
- 1 way communication with the help of Chat box.
Video Conferencing - It is connecting the International/Indian KOL with local doctors/KOLs across regions, for group viewing by the doctors.
Real time interaction with the international KOL - 2 way communication, where doctors can directly communicate with the KOL.
- Various benefits of all the above web based CMEs can be archived for later viewing. We also provide multiple touch points for sales force to market/hype the activity with cost effective mode
Identify, recruit faculties of reputed SMEs
- Presentations are delivered focusing on the particular therapy area and molecules in focus, across multiple cities by using the local subject matter expert
- Through these kind of activities, we provide multiple touch points based marketing collaterals to support the programs including event management
- Identify, recruit International faculties of reputed SMEs
- Presentations road show made across multiple cities by the International expert who has been actively involved in landmark clinical trials.
- Through these kind of activities, we provide multiple touch points based marketing collaterals to support the programs including event management Identify, recruit Indian medical societies
- Identify, recruit Indian medical societies
- Presentations road show made across multiple cities by the Indians expert who has remarkable reputations under the aegis of the selected society
- We also help to get the Indian credit points to the participants.
- Through these kind of activities, we provide multiple touch points based marketing collaterals to support the programs including event management
- We also conduct Live CME workshops in the form residential format
- The Faculties and the delegates gets time to networking during the evening time post workshop time
- We have lot of global hospitals and Institutes to execute such workshops in their auditorium with Certifications
- Through these kind of activities, we provide multiple touch points based marketing collaterals to support the programs including event management.
Offering 360° solutions covering brand ambassador in correlation with brand strategic decisions. we can avail the celebrity for Product launch, corporate AV, Promo's , catalogue & brochures Anatomical Models
Scientific part models can be sourced depending on the requirement
WHO WE SERVE
HEALTHCARE PROFESSIONALS
Cims Drug Book 2019 Pdf Free Download
Clinicians are constantly looking to update their knowledge and find better ways to practice medicine. CIMS provides clinicians with access to high-quality medical knowledge and tools to help them make informed clinical decisions.
PATIENTS
Patients are becoming more proactive in managing their health. CIMS Health provides resources and advice on health-related issues and drug information, so individuals can enhance their lifestyle.
HEALTHCARE INDUSTRY
To meet the healthcare needs of the community, pharmaceutical companies work with medical professionals to develop innovative products. With its unique marketing solutions, CIMS provides an excellent means to effectively communicate new knowledge to the healthcare community.
The MIMS Mobile app for Android™/ IOS™ is available for free.
For more information, visit www.mims.com/mobile-app
---------------------------------------------------------------------------------------------------------------------------------------------
Key features available in our app:
Drug Information
• Search for drug dosing information or specific drug interactions, and find the answers you need in seconds with our concise and comprehensive drug database.
• Based on locally-approved prescribing information, drug monographs are written and kept up-to-date by licensed healthcare professionals.
Disease & Condition Management Guidelines
• Voted most valuable online clinical resource by doctors in Asia.
• Review up-to-date disease management guidelines and be assured of reliable content fully substantiated by validated references and internationally-recognised researches, to enable you to make better-informed prescribing decisions.
Medical News & CME Updates
• Read the latest news available across various specialties in Asia through our renowned publications (Medical Tribune, JPOG, Oncology Tribune, etc), and keep your knowledge and skills current with changes in medicine.
Clinical Tools
• Access 29 clinical calculators and scoring tools such as the Body Mass Index (BMI) calculator and drug formulary, to facilitate effective clinical decision-making and support your practice.
• Developed and validated by a specialist team of doctors and pharmacists.
Multimedia
• The MIMS award-winning medical multimedia series is now accessible from the app.
• Watch insightful video interviews focusing on treatment options, disease management and latest updates by experts from various specialties, and upgrade your medical knowledge.
For more information, visit www.mims.com/mobile-app
If you have any feedback for us, you are welcome to email us at androidfeedback@mims.com
We would love to hear from you.
Cims Drug Book 2020 Pdf Download
Abstract
Background:
Drug information can be obtained from various sources such as National Formularies, drug package inserts (PI), other sources such as Monthly Index of Medical Specialities (MIMS), Current Index of Medical Specialities, and the information available with the regulators. Any variation in the information available in different sources can promote irrational drug use. In this study, we assessed this variation in a sample of commonly used drugs.
Mims Drug Book Pdf
Materials and Methods:
Fifty commonly used drugs were analyzed for any variation (both quantitative and qualitative) in information on indications as mentioned in commonly used drug information sources such as Central Drugs and Standards Control Organization (CDSCO) website, National Formulary of India (NFI), MIMS, and PI of medicines.
Results:
We observed a variation in average number of indications per drugs given in CDSCO (2.2 ± 0.25), NFI (3.51 ± 0.42), MIMS (2.98 ± 0.29), and PI (3.18 ± 3.52). The CDSCO and NFI did not contain information about indication for 10 and 17 drugs, respectively, while MIMS and PI contained information about all the selected drugs. A subset analysis was done for 24 such drugs which were mentioned in all the four sources and it was found that NFI had listed the maximum number of indications per drug (3.79 ± 0.53), followed by PI (3.08 ± 0.44), MIMS (3.04 ± 0.51), and CDSCO website (2.66 ± 0.37) and this difference was found to be statistically significant (P = 0.02). We also observed some gross qualitative variation regarding drug information given in different sources.
Conclusion:
Variation exists in the quantity and quality of information available on indications about drugs available in various sources. Necessary steps need to be taken to harmonize drug information available across various sources so as to provide reliable and uniform drug information thereby promoting rational drug use.

INTRODUCTION
Irrational and inappropriate use of drugs can lead to suboptimal clinical benefit and possible adverse drug reactions (ADRs).[1] Availability of clinically relevant, contemporary, and unbiased drug information goes a long way in promoting rational use of drugs. There are various sources of information which are utilized by treating physicians for accessing relevant drug information such as their indications, ADRs, contraindications, and special precautions. Drug information is usually sourced from National Formularies (e.g. National Formulary of India (NFI), British National Formulary), package inserts (PIs) of drugs, drug compendia such as Monthly Index of Medical Specialities (MIMS), Current Index of Medical Specialities (CIMS), and medical textbooks.[,3] The drug information available in various sources should be uniform, reliable, and conforming to the regulatory label of the drug. Every drug is approved for a specific indication(s) by drug regulator of the country, and these indication(s) is/are known as approved indication(s) of the drug.[,] If the drug is used for any indication other than the approved indication, it is known as off-label use of the drug. Approved indications of all the approved drugs are available with Central Drugs and Standards Control Organization (CDSCO), which is the national drug regulator in India. It has been observed that there is variation in the quantity and quality of information mentioned in different drug information sources and a single credible benchmark is lacking. Such variation not only deprives the medical fraternity from accessing reliable drug information but can also promote off-label and irrational drug use leading to increased incidence of adverse reactions and possible treatment failure.[,] We planned the present study to assess this variation in a sample of randomly selected drugs with respect to their indications given in various sources of drug information.
MATERIALS AND METHODS
We identified 50 commonly used drugs belonging to different groups (e.g. antimicrobials, antihypertensives, analgesics, antiulcer and antiemetics, anticancers, antidiabetics, and antiobesity drugs and also lifestyle drugs such as sildenafil) used in various specialty and superspecialty centers of our hospital. These drugs were chosen on the basis of prescription pattern in the hospital. Two senior residents (D.M. Clinical Pharmacology students) and one PhD student collected and analyzed the PIs of the selected drugs. These drugs were then analyzed for any variations in the information on a number of indications as mentioned in commonly used drug information sources (CDSCO web site, NFI, MIMS, and PIs). The following parameters were assessed.
The number of drugs out of the selected 50, whose indication information was missing in different sources.
Total number of indications given in different sources, in respect of these 50 drugs.
Average number of indications per drug mentioned in different sources.
After doing the above assessments, we did a subset analysis in respect of:
Only those drugs whose indications were given in CDSCO website list.
In the next step, we compared only those drugs whose indications were mentioned in all the four sources.
We also looked upon gross qualitative differences existing across various sources of drug information used in this study.
Statistical analysis
The data were represented as mean ± standard error of mean and median (range). To find the difference between different sources, data were statistically analyzed by applying Friedman Test using Graph Pad Instat (trial version) software.
RESULTS
PIs of all the 50 selected drugs were collected, and they had information about indications which was included in the analysis for comparison. Only MIMS contained information about all the 50 drugs. CDSO and NFI had information about 40 and 33 drugs, respectively. The number of indications per drug was variable in all these four sources. The details of this information are given in Table 1.
A subset analysis was done in respect of only those 40 drugs which were available in CDSCO. NFI was excluded from this analysis as this source had information of only about 24 of these 40 drugs. Hence, this analysis included only three sources (CDSCO, MIMS, and PI). In respect of these 40 drugs, the PI had listed maximum number of indications (2.95 indications/drug), followed by MIMS (2.70 indications/drug) and CDSCO website (2.20 indications/drug). We found that the difference in a number of indications given in these three sources was not statistically significant (P = 0.07) [Table 2].
Table 2
Indication information available in CDSO, MIMS, and PI
To include NFI as well, a subset analysis was done in respect of those 24 drugs information about which were available in all the four sources including NFI. We found that NFI had listed maximum number of indications (3.79 indications/drug), followed by PI (3.08 indications/drug), MIMS (3.04 indications/drug), and CDSCO website (2.66 indications/drug). This difference in the number of indications was statistically significant (P = 0.02) [Table 3].
Table 3
Indication information available in all four sources
Qualitative differences
After the quantitative comparison, we identified any gross qualitative mismatch in information across these four sources. Following gross discrepancies are observed:
Of the 40 drugs mentioned in CDSCO, only broad single indication is mentioned in respect of some drugs (e.g. amphotericin B-febrile neutropenia in cancer patients; sodium valproate: All forms of epilepsy; torsemide: Diuretic; fluoxetine: For treatment of depression). It is apparent that such abridged information only reflects the broad use of the drug without providing more specific and relevant information to the prescribing physicians.
Labetalol is one of the preferred drugs for treatment of pregnancy induced hypertension, and its oral administration is considered as safe and effective as methyldopa.[,] However, as per the CDSCO website, it is indicated for the treatment of all forms of hypertension except hypertension of pregnancy, whereas MIMS and PI mentioned hypertension in pregnancy as one of the indications of labetalol.
The CDSCO site mentions the treatment of depression as the only indication for fluoxetine, whereas MIMS and PI mention other indications such as obsessive compulsive disorder, bulimia nervosa also. Surprisingly, NFI lists out the maximum indications for fluoxetine which includes premenstrual disorder, anorexia nervosa, and Parkinson's disease as well over and above the indications given in other three sources.
In respect of tablet levofloxacin, CDSCO mention prostatitis as the only indication, whereas MIMS and PI includes more indications.
For tablet topiramate, the CDSCO site mentions it only as an antiepileptic, whereas other sources go on to describe the type of epileptic disorders for which it is indicated. In addition, PI mentions prophylaxis of migraine as one of its indication.
Besides above variations, some minor typographical errors were also noticed in information provided in CDSCO, e.g., gabapentin being indicated for naturopathic pain instead of neuropathic pain; febuxostat for treatment of chronic hyperuricemia in conditions where urate depression has already occurred instead of urate deposition; sildenafil for pulmonary osterial hypertension and not for pulmonary arterial hypertension.
DISCUSSION
In India, Drugs Controller General of India is the drug regulatory authority who is responsible for granting approval and marketing permission of drugs in our country. The drugs are approved by drug regulator of any country for specific indications in specified dosage, which is known as the labeling information of that particular drug. However, the actual use of the drug in clinical practice may vary and may not be according to its labeling information at times. Treating physicians can and do use drugs for any indication based on his/her clinical judgment. For example, metformin is used for the treatment of polycystic ovarian disease which is not its approved indication. Such a use of an approved drug is known as off-label use. Off-label use of a drug is not illegal, however, it may be irrational or unscientific.[]
In order to promote the rational and scientific use of drugs, it is important that relevant information about any drug, namely its indications, contraindications, and dosage are readily available to the prescribing physicians. Some common sources which are relied upon by the prescribing physicians to access drug information are NFI, PI, commercially published drug compendia such as MIMS and CIMS.[,3] The information available in such sources should confirm with the labeling information approved by the drug regulator of that country so that there is uniformity in decision making irrespective of the source(s) of information.
We undertook this study to assess the quantity and quality of drug information available in various sources and compared it with the labeling information of the drug as provided in the CDSCO website, which is the regulatory benchmark. The information about indications was taken as the sole benchmark of overall drug information, and various sources were compared on the basis of this parameter. It was observed that no information about indications of 10 out of the selected 50 drugs was available on the CDSCO website. Of these 10 drugs, 8 (tablet metformin, tablet acetazolamide, tablet verapamil, tablet carbamazepine, tablet spironolactone, tablet nitrofurantoin, tablet methotrexate, and injection isoprenaline) drugs were not even mentioned and the rest 2 (tablet propranolol and tablet prazosin) were enumerated without any mention of indications. This can be explained on the basis of the fact that CDSCO website only contains information from 1971 onward, and all these missing drugs are fairly old.
NFI, on the other hand, did not contain information about 17 drugs. NFI is mandated to include all the drugs in National List of Essential Medicines and some other commonly used drugs.[10] The most of the missing drugs (such as risedronate, erythropoietin, and faropenem) are used only in specialized centers and hence shall not be a part of NFI. However, the indication information about some commonly used drugs such as prazosin, levofloxacin, and chlorthalidone was also missing in NFI.
MIMS is a commercially available drug information compendium and was found to contain more number of indications as compared to the regulatory benchmark. Since this source is commonly utilized for seeking drug information, such a discrepancy may encourage off-label, and sometimes irrational, use of drugs.
PIs were also found to contain maximum indications over and above the ones mentioned in regulator's website. In our country, PIs are to be provided to the regulator at the time of registration. However, providing and publishing PIs along with drug packages in neither regulated nor mandatory.[11] In fact, all the pharmaceutical companies do not provide PIs. However, when available, listing any indication in PI which is not in consonance with the regulator's website may again lead to off-label and possible irrational drug use.[12,]
On the basis of aforementioned, it can be safely inferred that the CDSCO website does not contain information about some commonly used drugs even while these drugs are marketed in India. These 10 drugs are part of 50 commonly prescribed drugs as identified for this study. If all the drugs marketed in India are checked, this number may increase. Such a deficiency has the portent to compromise the robustness of regulatory benchmark and may lead to confusion regarding the appropriate use of drugs.
This study highlights the discrepancies in drug information available in various sources by taking a representative sample of commonly used drugs. To the best of our knowledge, this study, though parsimonious in design, is the first such attempt to address this issue.
These 50 drugs were identified on the basis of the prescription pattern in our hospital which is a tertiary care center. One limitation of this study, is that, this prescription pattern may vary from one hospital to other, and a more broad based selection criteria for identifying drugs may be desirable.
CONCLUSION
Variation exists in the quantity and quality of information on indications about drugs available in various sources. PI and MIMS provide information on indications about maximum number of drugs. However, this information does not conform to regulatory benchmark all the time. Information about a number of drugs was not available in CDSCO website and NFI. CDSCO website is the regulatory benchmark and requires updating so as to provide a sound and reliable reference regarding drug information for all the stakeholders. Further studies involving large sample of drugs and more variables (such as side effect profile, dosage information, information on drug interactions, and special precautions and contraindications) are required to further elucidate the issue of variation in drug information.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
Cims Drug Book 2018 Pdf Download
Cims Drug Book 2018 Pdf Free Download
Cims Drug Book 2017 Pdf Download
